Researchers work alongside robotic automation systems in an AI-connected cloud laboratory optimizing protein synthesis experiments through closed-loop iteration. Image Source: ChatGPT-5.2
GPT-5 Reduces Cell-Free Protein Synthesis Costs by 40% in Autonomous Lab Collaboration with Ginkgo Bioworks
OpenAI has demonstrated that GPT-5 can reduce the cost of cell-free protein synthesis (CFPS) by 40% when connected directly to a robotic cloud laboratory operated by Ginkgo Bioworks. In a closed-loop autonomous system, GPT-5 designed experiments, executed them remotely through lab automation, analyzed results, and iteratively refined reaction compositions across six experimental rounds.
During the project, more than 36,000 unique CFPS reaction combinations were tested on 580 automated plates. After being provided access to a computer and web browser, along with relevant research papers, computational tools, and laboratory execution systems, GPT-5 established a new low-cost benchmark for CFPS within three rounds of experimentation.
The collaboration demonstrates how frontier AI models can operate inside physical laboratory workflows, not just digital environments.
AI systems have advanced rapidly in fields like mathematics and physics, where ideas can often be evaluated computationally. Biology is different. Progress depends on physical experiments that require time, materials, and laboratory infrastructure. Connecting AI directly to automated laboratory systems begins to change that constraint.
In many areas of life science, the primary bottleneck is iteration speed. Autonomous laboratories increase experimental throughput, allowing thousands of reaction variations to be tested more quickly than traditional manual workflows.
In earlier work, GPT-5 demonstrated the ability to improve wet-lab protocols through closed-loop experimentation. This study extends that approach to cost optimization in protein production.
Key Takeaways: GPT-5 Autonomous Lab Protein Cost Reduction
GPT-5 reduced cell-free protein synthesis (CFPS) costs by 40% in a live autonomous lab environment.
The system tested over 36,000 reaction compositions across 580 automated plates.
GPT-5 operated in a closed-loop cycle: experiment design, robotic execution, data analysis, and iteration.
Reagent cost efficiency improved by 57%.
Results were demonstrated on the sfGFP protein within a defined CFPS system.
Human oversight remained required for lab protocol execution and safety validation.
WHY CELL-FREE PROTEIN SYNTHESIS (CFPS) MATTERS FOR BIOTECHNOLOGY
Cell-free protein synthesis (CFPS) produces proteins without growing living cells. Instead of inserting DNA into cells and waiting for biological expression, CFPS runs protein-production machinery in a controlled mixture. This allows researchers to prototype and test biological designs rapidly.
Proteins are central to modern biology. They underpin many medicines and diagnostics, and in industrial settings they act as enzymes that make chemical processes cleaner and more efficient. Proteins are even found in everyday products like laundry detergent, where they help break down stains. Lowering protein production costs can reduce barriers to experimentation and shorten development timelines across biotechnology and industrial biology.
However, optimizing cell-free protein synthesis (CFPS) is complex and expensive at scale. The system involves interacting ingredients: the DNA template encoding the protein, cell lysate — the mixture of cellular machinery extracted from cells — and numerous biochemical components ranging from energy sources to salts and buffering agents. Because the reaction depends on multiple interdependent variables, small adjustments can produce disproportionate and sometimes unexpected effects on protein yield.
Standard CFPS formulations and commercial kits are typically priced for human-paced experimentation. Autonomous laboratories, however, can run thousands of reactions in the time a human team might run dozens. At that scale, the cost of reagents, or the chemical components consumed in each reaction, becomes the limiting factor. Exploring the full combinatorial space manually is labor-intensive, and prior cost reductions have tended to be incremental rather than transformative.
HOW GPT-5 CONNECTED TO GINKGO BIOWORKS’ CLOUD LABORATORY
To address this optimization challenge, GPT-5 was paired with Ginkgo Bioworks’ cloud laboratory, forming a closed-loop autonomous system for CFPS optimization.
The workflow operated as follows:
GPT-5 proposed experimental designs.
The robotic laboratory executed the experiments remotely.
Data was returned to GPT-5.
The model analyzed results and proposed the next iteration.
The cycle repeated across six rounds.
Strict programmatic validation ensured that AI-designed experiments were physically executable within the automation platform. This prevented “paper experiments” — designs that appear plausible in text but cannot be carried out in a robotic workflow.
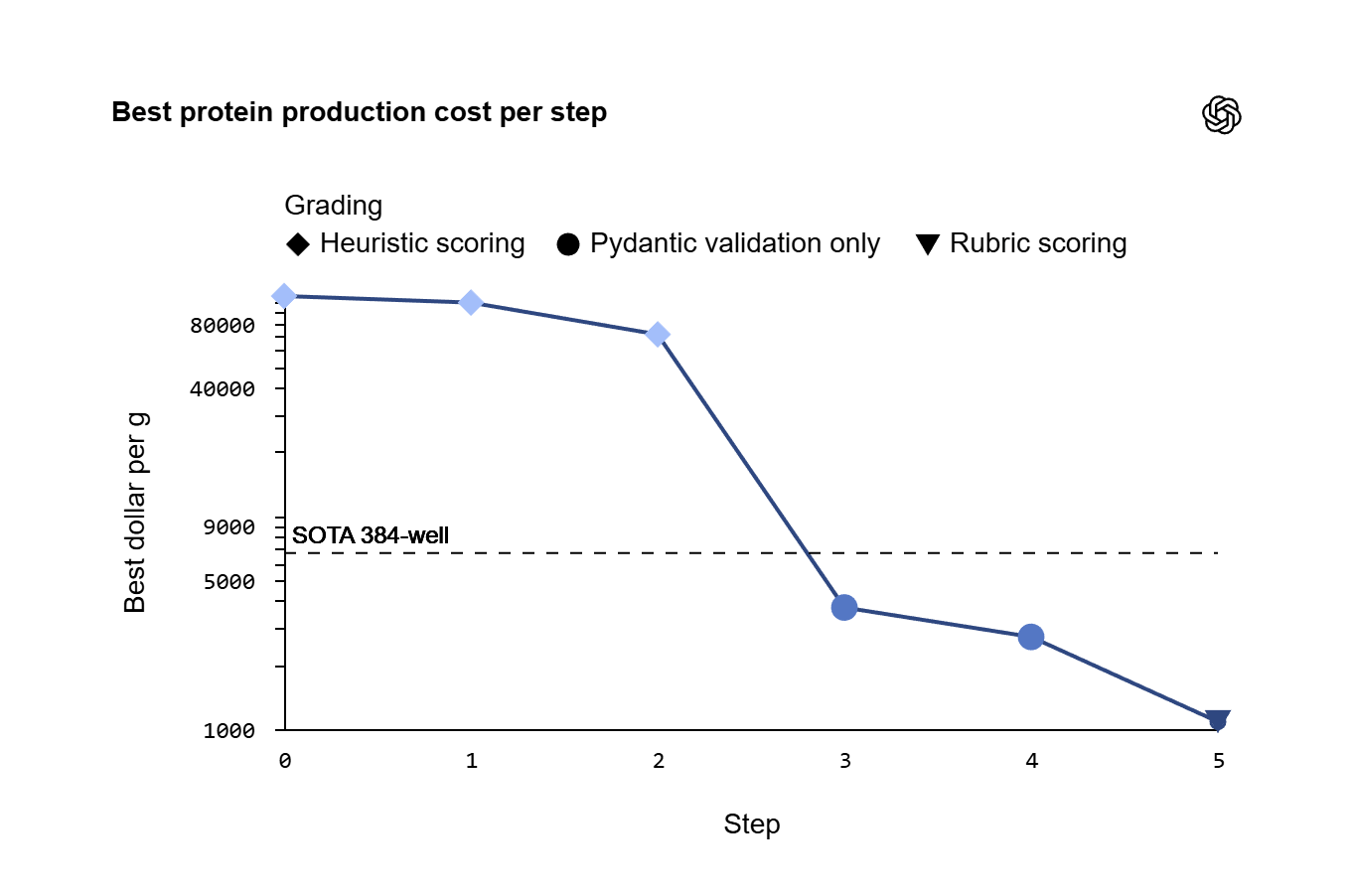
Across the full run, the system executed more than 36,000 CFPS reactions on 580 automated plates. Over a two-month period, and after being provided access to a computer and web browser along with relevant research papers and computational tools, GPT-5 established a new low-cost benchmark for CFPS within three rounds of experimentation — achieving a 40% reduction in protein production cost compared to the best prior baseline.
CLOSED-LOOP AI ITERATION IMPROVES CFPS YIELD AND COST
In biological systems, individual experiments are often noisy, and meaningful patterns only emerge through repeated iteration at scale. Automation allows thousands of reaction variations to be tested quickly, making it easier to identify patterns that would be missed in smaller, manual experiments.
By running more than 36,000 different reaction combinations, GPT-5 tested far more possibilities than a human team could realistically attempt. It found specific ingredient mixes that delivered better protein yield without increasing cost — particularly adjustments to buffering agents (which help maintain stable chemical conditions), energy components that power the reaction, and polyamines, small molecules that support protein production.
Notably:
GPT-5 proposed ingredient combinations in ratios and concentrations that had not previously been tested together in this specific high-throughput plate format.
Small automated plate experiments often have lower oxygen levels and less mixing than larger test-tube setups, which can reduce protein production. Many of the high-performing mixtures GPT-5 identified continued to work well despite those constraints.
Small adjustments to buffering agents, energy regeneration components, and polyamines — ingredients that help stabilize and power the reaction — meaningfully improved protein yield without adding significant cost.
Because lysate and DNA are the most expensive inputs in CFPS, increasing protein output per unit of those materials had the greatest impact on overall production cost.
Rather than relying on a single dramatic change, the cost reductions came from identifying combinations that consistently performed well under real automation conditions.
COST AND YIELD IMPROVEMENTS ACROSS GPT-5 EXPERIMENTAL ROUNDS
Across iterative steps, performance improved in both yield and cost efficiency.
The graphs show that as the experiments progressed, GPT-5 was able to produce more protein at a lower cost. With each round of testing, the cost per gram of protein decreased, outperforming both earlier baselines and prior high-throughput laboratory methods.
These improvements reflect iterative refinement rather than a single breakthrough experiment.
LIMITATIONS: SCOPE AND GENERALIZATION
The results were demonstrated on:
One protein (sfGFP)
One specific cell-free protein synthesis system
Generalization to other proteins, scales, and CFPS systems remains to be validated.
Additionally:
Oxygen levels and the physical setup of the reaction — including container size and mixing conditions — can significantly affect how much protein is produced. These factors often differ between small automated plates and larger laboratory setups.
Some of the improvements identified in this study may depend on those specific conditions, meaning further testing is needed to determine how well they generalize to other scales or systems.
Human operators were still required for protocol adjustments, reagent preparation, and practical laboratory management.
While GPT-5 autonomously designed and analyzed experiments, the physical execution of laboratory work continues to rely on experienced scientific oversight.
BIOSECURITY AND SAFEGUARDS IN AI-DRIVEN LABORATORY RESEARCH
OpenAI indicated that connecting AI systems to wet-lab environments, where experiments are physically carried out using biological materials, raises important biosecurity considerations. While autonomous laboratories can accelerate experimentation, biological research still requires careful oversight, validation, and responsible safeguards.
The company evaluated this work under its Preparedness Framework, which is designed to assess and mitigate potential risks associated with advanced AI capabilities. OpenAI stated that safeguards are implemented at both the model level (how the system reasons and generates outputs) and the system level (how it is deployed and connected to laboratory infrastructure).
Researchers emphasized that while models can propose and analyze experiments, biological progress still depends on real-world testing and scientific judgment. Evaluating and reducing potential biosecurity risks remains part of the ongoing development process as lab-in-the-loop systems expand into additional biological workflows.
Q&A: UNDERSTANDING GPT-5’S AUTONOMOUS LAB PROTEIN OPTIMIZATION
Q: What did GPT-5 achieve in this study?
A: GPT-5 reduced the cost of cell-free protein synthesis (CFPS) by 40% through closed-loop experimentation inside an automated cloud laboratory operated by Ginkgo Bioworks.
Q: How did GPT-5 interact with the laboratory?
A: The model designed experimental batches, the robotic cloud lab executed them, results were returned to GPT-5, and the model analyzed the data to propose the next round of experiments.
Q: What scale of experimentation was involved?
A: More than 36,000 unique CFPS reaction compositions were tested across 580 automated plates over six rounds of iteration.
Q: What protein was used in the experiments?
A: The system optimized production of sfGFP, a commonly used fluorescent protein in biological research.
Q: Does this generalize to all protein production systems?
A: No. The results were demonstrated on one protein and one CFPS system. Broader validation is still required.
Q: Why is this important for biotechnology?
A: Lowering protein production costs can accelerate drug development, diagnostics, industrial enzyme production, and other biotechnology applications by reducing the cost of experimentation and scaling.
WHAT THIS MEANS: AI MODELS ENTER CLOSED-LOOP BIOLOGICAL OPTIMIZATION
This demonstration goes beyond lowering the cost of one protein. It shows that a frontier AI model can operate inside a real laboratory feedback loop — designing experiments, executing them through automation, analyzing results, and refining its own strategy at scale.
Who should care
Biotechnology companies, synthetic biology researchers, pharmaceutical R&D teams, and industrial enzyme developers evaluating high-throughput experimentation platforms will find this development particularly relevant. Organizations investing in laboratory automation may view closed-loop AI systems as a potential lever for accelerating research and reducing production costs.
Why it matters now
In many life science workflows, the limiting factor is iteration speed. Experiments take time, materials, and human coordination. When AI systems can design experiments, connect directly to robotic lab infrastructure, analyze outcomes, and propose next steps, the constraint shifts from human bandwidth to system throughput.
That shift has practical implications for development timelines, cost structures, and how research priorities are set. The threshold demonstrated here is not simply improved protein yield — it is the ability of an AI system to participate in sustained, large-scale biological optimization.
What decision this affects
For biotechnology and industrial biology organizations, this development may influence decisions about investing in automated cloud laboratories, integrating AI into experimental workflows, and building infrastructure for scalable experimentation. It also shapes how teams structure human oversight, safety controls, and validation processes in AI-enabled research environments.
While the results remain bounded to a specific protein and CFPS system, they provide an early indication that autonomous laboratory iteration can materially influence production economics under defined conditions.
If similar approaches generalize to other proteins and biological workflows, this model of AI-driven experimentation could accelerate the development of protein-based medicines, diagnostics, industrial enzymes, and consumer products — reducing costs and shortening the path from laboratory discovery to real-world application.
Sources:
OpenAI – GPT-5 Lowers Protein Synthesis Cost
https://openai.com/index/gpt-5-lowers-protein-synthesis-cost/OpenAI – Accelerating Science with GPT-5
https://openai.com/index/accelerating-science-gpt-5/OpenAI – Accelerating Biological Research in the Wet Lab
https://openai.com/index/accelerating-biological-research-in-the-wet-lab/Ginkgo Bioworks
https://www.ginkgo.bio/PubMed (National Library of Medicine)
https://pubmed.ncbi.nlm.nih.gov/21520017/Nature Communications
https://www.nature.com/articles/s41467-020-15798-5Nature Communications
https://www.nature.com/articles/s41467-025-58139-0OpenAI – Updating Our Preparedness Framework
https://openai.com/index/updating-our-preparedness-framework/
Editor’s Note: This article was created by Alicia Shapiro, CMO of AiNews.com, with writing, image, and idea-generation support from ChatGPT, an AI assistant. However, the final perspective and editorial choices are solely Alicia Shapiro’s. Special thanks to ChatGPT for assistance with research and editorial support in crafting this article.